
Ionic Bonding

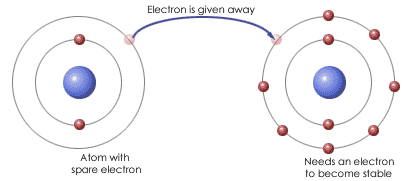
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds.
The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged).
This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH4+ or SO42−.
In simpler words, an ionic bond is the transfer of electrons from a metal to a non-metal in order for both atoms to obtain a full valence shell.
Inter-molecular forces
Intermolecular forces (IMFs) are the forces which mediate interaction between molecules. They also include forces of attraction or repulsion which act between molecules and other types of neighboring particles (e.g., atoms or ions).
The intermolecular forces form the attractions between the metal and the non-metal.
CITATION:
Structure and Bonding in Chemistry. (n.d.). Retrieved December 27, 2016, from http://www.chm.bris.ac.uk/pt/harvey/gcse/ionic.html
BBC - GCSE Bitesize: Ionic bonding. (n.d.). Retrieved December 27, 2016, from http://www.bbc.co.uk/schools/gcsebitesize/science/add_gateway_pre_2011/periodictable/ionicbondingrev1.shtml
BBC - GCSE Bitesize: Ionic bonding. (n.d.). Retrieved December 27, 2016, from http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa/bonding/ionic_bondingrev4.shtml

